About the MOOSE Study
The MOOSE study aims to find out whether methotrexate injections are more effective than tablets in controlling rheumatoid arthritis (RA). MOOSE will also look at whether methotrexate injections are value for money for the NHS. Part of the study will look at how acceptable to patients methotrexate tablets and injections are as a first line treatment for rheumatoid arthritis.
Methotrexate is usually prescribed as weekly tablets for treating RA. Some people may have side effects such as sickness and they may then be prescribed weekly injections of methotrexate. These injections are self-administered to just below the skin in the thigh or the tummy. MOOSE will help to find out whether it is best to give tablets or injections as the first option. Those who take part will be randomised into one of two study groups:
- Methotrexate injections
- Methotrexate tablets
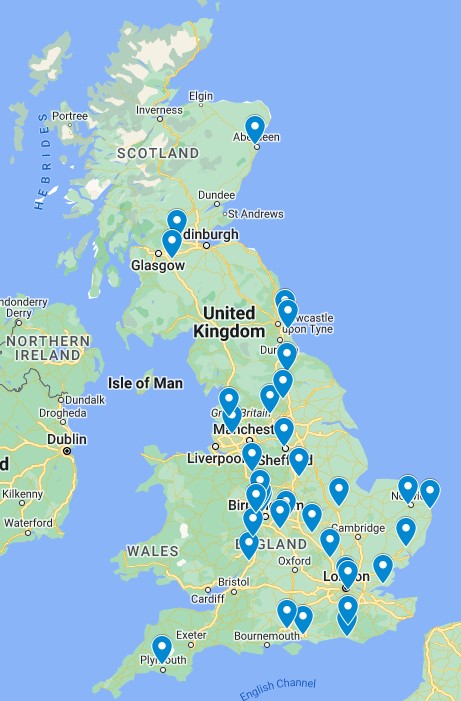
We will be recruiting 386 participants to take part in the MOOSE study. Potential participants will be RA patients who are attending the rheumatology clinic for the first time, and being offered methotrexate to help with their RA symptoms. The hospital clinics, and people taking part in the study will be from England, Scotland and Wales, and from a variety of large city-based hospitals as well as smaller more rural hospitals.
The MOOSE study is a pragmatic study, which means that it has been designed to follow standard clinical practice, and participants will be cared for in the same way as any rheumatoid arthritis patient usually would be. This allows all participants to receive the treatments they need, as and when they need them, with the help of their usual rheumatology team at the hospital clinic.
Throughout the study we will be following up participants progress at their clinic visits, and using questionnaires asking about their arthritis, ability to do daily activities and work, well-being, fatigue, and mental health. We will also give participants the opportunity to talk about their arthritis in one-to-one discussions designed to inform us about the acceptability of the treatments.
At the end of the study, the results will be shared with doctors, nurses, people with RA and patient charities such as the National RA Society. We will engage with NICE and the British Rheumatology Society to update national guidelines for treating RA.
Progress
Recruitment has now finished, and we have 388 participants in the study. Thank you to everyone involved, all participants and site staff, we are very grateful for your time and hard work.
MOOSE study sites across England and Scotland